- Best Practices New Normal
- Digital Dentistry
- Data Security
- Implants
- Catapult Education
- COVID-19
- Digital Imaging
- Laser Dentistry
- Restorative Dentistry
- Cosmetic Dentistry
- Periodontics
- Oral Care
- Evaluating Dental Materials
- Cement and Adhesives
- Equipment & Supplies
- Ergonomics
- Products
- Dentures
- Infection Control
- Orthodontics
- Technology
- Techniques
- Materials
- Emerging Research
- Pediatric Dentistry
- Endodontics
- Oral-Systemic Health
5Ws* Urbanek TMJ Device
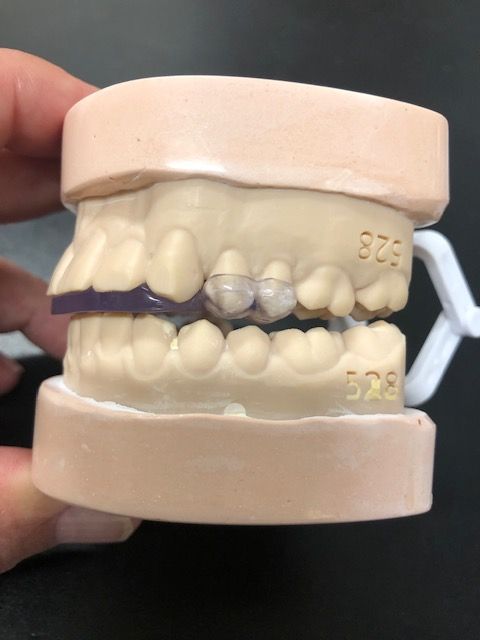
This device and protocol are the only nonsurgical, custom CAD-CAM, 3D-printed resin solution that alleviates the inflammation of the temporomandibular joint.
5Ws* Urbanek TMJ Device | Credit: © Urbanek TMJ Device and Protocol
Information provided by Urbanek TMJ Device and Protocol.
What
Urbanek TMJ Device and Protocol
Who
Urbanek TMJ Device and Protocol
888-242-7460
When
Whenever patients are suffering from temporomandibular joint disorder (TMD). Results have been successful with patients in as few as 2 office visits, the company states.
Where
Designed by Anthony Urbanek, DDS, MD, MS, the Urbanek TMJ Device and Protocol officially launched its commercial operations in North America, offering dentists a new way to treat the more than 10 million TMD sufferers in the United States. Clinicians who become licensed providers gain access to all the tools needed to successfully grow their practice and become a trusted TJM Device and Protocol.
Why
Developed by an oral maxillofacial surgeon with over 40 years of experience, the Urbanek TMJ Device and Protocol is the only nonsurgical, custom computer-aided design/computer-aided manufacturing, 3D-printed resin device that alleviates the inflammation of the temporomandibular joint (TMJ). Unlike a night guard, this FDA-cleared medical device was specifically designed to treat the root cause of TMD.
The How*
The device, customized to each patient, unloads the joint just like a set of crutches unloads an inflamed knee. The patient starts by wearing the device 24/7 for 2 months, and then at nighttime only for the rest of their life. That works for about 85% of the patients, reportedly, and the company has completed 4000 patient cases with a success rate of 95%, which indicates what can happen if they follow the protocol.